The first nuclear production facility was built at the Hanford Site in 1943 as part of the Manhattan Project to support the production of plutonium for the first atomic weapons. From 1944 to1980, the facilities of the Hanford Site were involved in the production of nuclear fuel. The fabrication of natural and slightly enriched uranium into fuel elements for nuclear reactors in Hanford’s 300 Area, and the reprocessing of irradiated fuel in Hanford’s 200 Area to obtain plutonium and other useful radioisotopes, generated large volumes of radioactive waste containing uranium, among other constituents. This waste was stored in underground single-shell tanks, many of which have leaked contaminants into the surrounding soil. An estimated 200,000 kg of uranium was released into the ground at the 200 East and 200 West Areas. These leaks influenced the vadose zone by creating a potential source for groundwater contamination and risk to receptors through water uptake from contaminated wells or discharge to surface water. Despite extensive remediation efforts initiated in the early 1990s, uranium groundwater plumes identified in multiple locations around the site have persisted for many years. The protection of water resources from contaminated groundwater resulting from operations at the Hanford Site is a key element of the overall Hanford cleanup program.
As part of an innovative remediation effort, the Hanford Site plans to inject polyphosphate into the subsurface to target uranium contamination in the vadose zone, with the objective of reducing the potential for uranium mobility. The Applied Research Center at Florida International University is performing research to help quantify the long-term protection from uranium that can be expected from this remediation treatment.
An original manuscript, resulting from this research and titled “Comparison of the Kinetic Rate Law Parameters for the Dissolution of Natural and Synthetic Autunite in the Presence of Aqueous Bicarbonate Ions,” was accepted for publication in the peer-reviewed journal Chemical Geology on May 24, 2013. Authors from the Applied Research Center at Florida International University (Dr. Ravi Gudavalli, Dr. Yelena Katsenovich, Dr. Leonel Lagos, Dr. Berrin Tansel, and DOE Fellow Melina Idarraga) collaborated with a scientist at Pacific Northwest National Laboratory (Dr. Dawn Wellman) to develop this manuscript based on the results from the single-pass flow-through experiments that were conducted to estimate the rate of uranium release from calcium meta-autunite as a function of bicarbonate (0.0005 to 0.003M) in a range of pH (6-11) and temperature (5-60oC). The manuscript also quantifies the kinetic rate law parameters for Ca-autunite dissolution and provides details and explanations on the effect of bicarbonate/carbonate ions on the uranium release reactions mechanism. Since the planned injection of polyphosphate into the subsurface at Hanford is expected to cause the formation of autunite, FIU’s research will help predict the long-term effectiveness of the planned remediation effort.
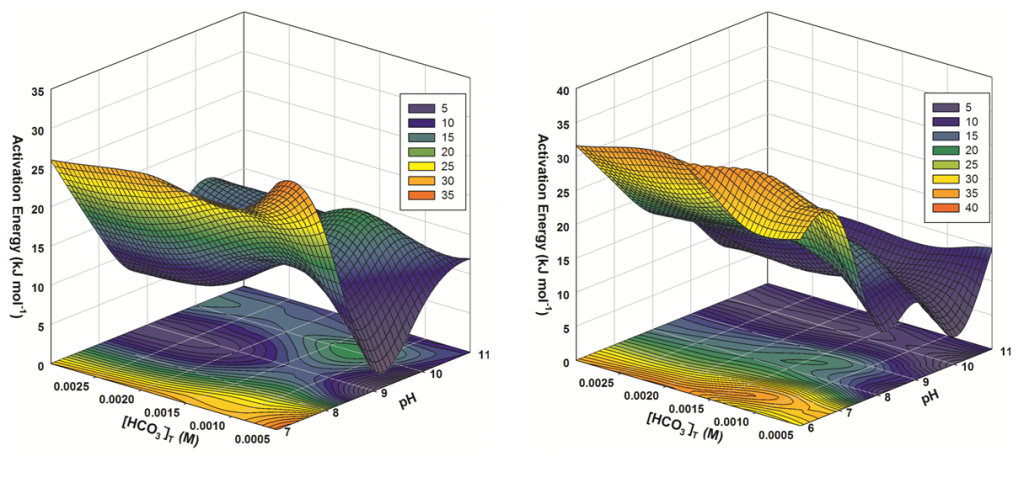
Figure 2. 3D representations of the activation energy for calcium autunite (left) and sodium autunite (right) dissolution at varying pH and concentrations of bicarbonate solutions.
Additional FIU peer-reviewed publications based on the research work they have performed under the FIU-DOE Cooperative Agreement (DE-EM-0000598) during the past year include:
These publications and additional technical reports and papers can be found on the website dedicated to the applied research being performed under the Cooperative Agreement between the U.S. Department of Energy (DOE) Office of Environmental Management (EM) and FIU: http://doeresearch.fiu.edu. Additional information can be obtained by contacting Dr. Leonel Lagos (Principal Investigator, Director of Research) at (305) 348-1810 or lagosl@fiu.edu.
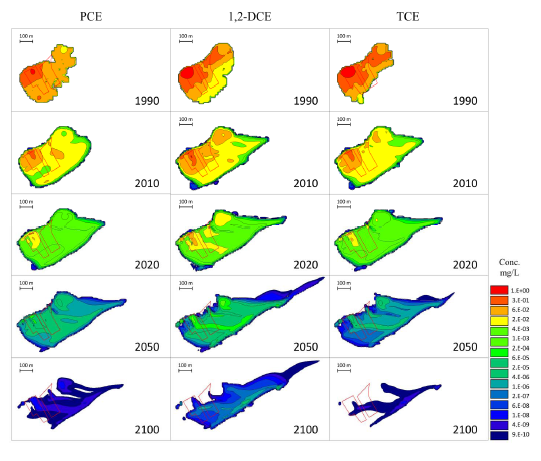
Figure 1. Volatile organic compound (VOC) plume migration in the subsurface computed for 100 years at the Old Salvage Yard (OSY), Y-12 National Security Complex.