Sponsor – DOE
FIU’s Applied Research Center (ARC) is supporting the U.S. Department of Energy’s Hanford Site in developing a strategy to improve the efficiency of the uranium stabilization process through polyphosphate injection technology.
Tripolyphosphate injected in aqueous solutions in order to sequester uranium, undergoes hydrolysis to orthophosphate forms, which serve as readily available nutrients for the various micro-organisms that thrive under these specific conditions and may even lead to an increase in their growth. The presence of rapidly adapting bacterial populations in sediment could strongly influence the migration/dissolution of uranium by dissolution and desorption due to the secretion of protons and various ligands. Therefore, understanding the role of bacteria in phosphate remediation technology and the interactions between meta-autunite and the microbes is very important. Of particular concern is the long-term stability of the sequestered uranium in the subsurface that may undergo subsequent remobilization. This task is designed to investigate bacteria-U(VI) interactions under oxygen restricted and anaerobic conditions, and study the potential bicarbonate-assisted U(VI) release from autunite minerals and uranium reduction by bacterial cells.
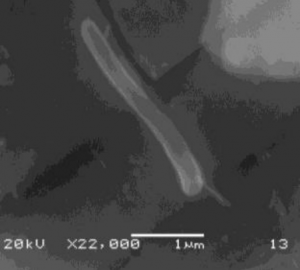
Rod shaped Shewanella oneidensis MR-1 on the surface of natural autunite viewed under Scanning Electron Microscopy
Objectives:
Benefits:
Accomplishments:
Sponsor - DOE FIU’s Applied Research Center (ARC) is supporting the U.S. Department of Energy’s Hanford Site in developing...
Sponsor - DOE FIU’s Applied Research Center (ARC) is supporting the U.S. Department of Energy’s Hanford Site in developing a...
Sponsor - DOE FIU’s Applied Research Center (ARC) is supporting the U.S. Department of Energy’s Savannah River Site in...
Sponsor - DOE This task supports US DOE EM-13 in developing plans for improving active remediation systems to improve performance...